Applications of focused ultrasound in the brain: from thermoablation to drug delivery
Focused ultrasound (FUS) is a disruptive medical technology, and its implementation in the clinic represents the culmination of decades of research. Lying at the convergence of physics, engineering, imaging, biology and neuroscience, FUS offers the ability to non-invasively and precisely intervene in key circuits that drive common and challenging brain conditions. The actions of FUS in the brain take many forms, ranging from transient blood–brain barrier opening and neuromodulation to permanent thermoablation. Over the past 5 years, we have seen a dramatic expansion of indications for and experience with FUS in humans, with a resultant exponential increase in academic and public interest in the technology. Applications now span the clinical spectrum in neurological and psychiatric diseases, with insights still emerging from preclinical models and human trials. In this Review, we provide a comprehensive overview of therapeutic ultrasound and its current and emerging indications in the brain. We examine the potential impact of FUS on the landscape of brain therapies as well as the challenges facing further advancement and broader adoption of this promising minimally invasive therapeutic alternative.
Key points
- Recent advances have led to a surge of interest in focused ultrasound (FUS) as a non-invasive, potentially disruptive tool for the most intractable neurological conditions.
- Magnetic resonance-guided FUS thermoablation has been approved for the treatment of essential tremor and tremor-dominant Parkinson disease and is being investigated in psychiatric applications as well as in chronic pain and epilepsy.
- Transient opening of the blood–brain barrier for drug delivery is a burgeoning field, with early human studies demonstrating a favourable safety profile as well as versatility across and scalability within a range of clinical indications.
- Future studies will investigate the delivery of established pharmaceuticals and novel therapies in combination with FUS blood–brain barrier opening.
- Emerging applications are also harnessing the myriad of ways in which FUS can interact with the CNS, including immune modulation and neuromodulation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
206,07 € per year
only 17,17 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Controlled noninvasive modulation of deep brain regions in humans
Article Open access 12 January 2024

Guiding and monitoring focused ultrasound mediated blood–brain barrier opening in rats using power Doppler imaging and passive acoustic mapping
Article Open access 30 August 2022
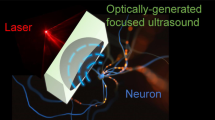
Optically-generated focused ultrasound for noninvasive brain stimulation with ultrahigh precision
Article Open access 03 November 2022
References
- Aubry, J.-F. et al. The road to clinical use of high-intensity focused ultrasound for liver cancer: technical and clinical consensus. J. Ther. Ultrasound1, 13 (2013). PubMedPubMed CentralGoogle Scholar
- Tempany, C. M. C., McDannold, N. J., Hynynen, K. & Jolesz, F. A. Focused ultrasound surgery in oncology: overview and principles. Radiology259, 39–56 (2011). PubMedPubMed CentralGoogle Scholar
- El-Hayek, Y. H. et al. Tip of the iceberg: assessing the global socioeconomic costs of Alzheimer’s disease and related dementias and strategic implications for stakeholders. J. Alzheimers Dis.70, 323–341 (2019). PubMedPubMed CentralGoogle Scholar
- Aldape, K. et al. Challenges to curing primary brain tumours. Nat. Rev. Clin. Oncol.16, 509–520 (2019). CASPubMedPubMed CentralGoogle Scholar
- Makin, S. The amyloid hypothesis on trial. Nature559, S4–S7 (2018). CASPubMedGoogle Scholar
- Lozano, A. M. et al. A phase II study of fornix deep brain stimulation in mild Alzheimer’s disease. J. Alzheimers Dis.54, 777–787 (2016). CASPubMedPubMed CentralGoogle Scholar
- Elias, W. J. et al. A randomized trial of focused ultrasound thalamotomy for essential tremor. N. Engl. J. Med.375, 730–739 (2016). This pivotal study led to regulatory approval of the first approved indication for MRgFUS thermoablation in the treatment of essential tremor. PubMedGoogle Scholar
- Bond, A. E. et al. Safety and efficacy of focused ultrasound thalamotomy for patients with medication-refractory, tremor-dominant Parkinson disease: a randomized clinical trial. JAMA Neurol.74, 1412–1418 (2017). This pivotal study led to regulatory approval of the second — and, to date, only other — approved clinical indication for MRgFUS thermoablation in the treatment of TDPD. PubMedPubMed CentralGoogle Scholar
- Leinenga, G., Langton, C., Nisbet, R. & Götz, J. Ultrasound treatment of neurological diseases — current and emerging applications. Nat. Rev. Neurol.12, 161–174 (2016). PubMedGoogle Scholar
- Gandaglia, G. et al. Effect of minimally invasive surgery on the risk for surgical site infections: results from the National Surgical Quality Improvement Program (NSQIP) Database. JAMA Surg.149, 1039–1044 (2014). PubMedGoogle Scholar
- Hynynen, K. & Jones, R. M. Image-guided ultrasound phased arrays are a disruptive technology for non-invasive therapy. Phys. Med. Biol.61, R206–R248 (2016). PubMedPubMed CentralGoogle Scholar
- Raymond, S. B. & Hynynen, K. Acoustic transmission losses and field alterations due to human scalp hair. IEEE Trans. Ultrason. Ferroelectr. Freq. Control.52, 1415–1419 (2005). PubMedGoogle Scholar
- Meyers, R. et al. Early experiences with ultrasonic irradiation of the pallidofugal and nigral complexes in hyperkinetic and hypertonic disorders. J. Neurosurg.16, 32–54 (1959). CASPubMedGoogle Scholar
- Nelson, E., Lindstrom, P. A. & Haymaker, W. Pathological effects of ultrasound on the human brain: a study of 25 cases in which ultrasonic irradiation was used as a lobotomy procedure. J. Neuropathol. Exp. Neurol.18, 489–508 (1959). CASPubMedGoogle Scholar
- Leksell, L. Echo-encephalography. I. Detection of intracranial complications following head injury. Acta Chir. Scand.110, 301–315 (1956). CASPubMedGoogle Scholar
- Jagannathan, J. et al. High-intensity focused ultrasound surgery of the brain: part 1 — a historical perspective with modern applications. Neurosurgery64, 201–210 (2009). PubMedPubMed CentralGoogle Scholar
- Guthkelch, A. N. et al. Treatment of malignant brain tumors with focused ultrasound hyperthermia and radiation: results of a phase I trial. J. Neurooncol.10, 271–284 (1991). CASPubMedGoogle Scholar
- Ram, Z. et al. Magnetic resonance imaging-guided, high-intensity focused ultrasound for brain tumor therapy. Neurosurgery59, 949–955 (2006). PubMedGoogle Scholar
- Hynynen, K. et al. Pre-clinical testing of a phased array ultrasound system for MRI-guided noninvasive surgery of the brain — a primate study. Eur. J. Radiol.59, 149–156 (2006). PubMedGoogle Scholar
- Clement, G. T. & Hynynen, K. A non-invasive method for focusing ultrasound through the human skull. Phys. Med. Biol.47, 1219–1236 (2002). CASPubMedGoogle Scholar
- Aubry, J.-F. & Tanter, M. MR-guided transcranial focused ultrasound. Adv. Exp. Med. Biol.880, 97–111 (2016). CASPubMedGoogle Scholar
- Haworth, K. J., Fowlkes, J. B., Carson, P. L. & Kripfgans, O. D. Towards aberration correction of transcranial ultrasound using acoustic droplet vaporization. Ultrasound Med. Biol.34, 435–445 (2008). PubMedGoogle Scholar
- Hynynen, K., Darkazanli, A., Unger, E. & Schenck, J. F. MRI-guided noninvasive ultrasound surgery. Med. Phys.20, 107–115 (1993). CASPubMedGoogle Scholar
- Jeanmonod, D. et al. Transcranial magnetic resonance imaging-guided focused ultrasound: noninvasive central lateral thalamotomy for chronic neuropathic pain. Neurosurg. Focus32, E1 (2012). PubMedGoogle Scholar
- Carpentier, A. et al. Clinical trial of blood–brain barrier disruption by pulsed ultrasound. Sci. Transl. Med.8, 343re2 (2016). PubMedGoogle Scholar
- Maimbourg, G., Houdouin, A., Deffieux, T., Tanter, M. & Aubry, J.-F. 3D-printed adaptive acoustic lens as a disruptive technology for transcranial ultrasound therapy using single-element transducers. Phys. Med. Biol.63, 025026 (2018). PubMedGoogle Scholar
- Haar, G. T. & Coussios, C. High intensity focused ultrasound: physical principles and devices. Int. J. Hyperth.23, 89–104 (2007). Google Scholar
- Mouratidis, P. X. E., Rivens, I., Civale, J., Symonds-Tayler, R. & Ter Haar, G. ‘Relationship between thermal dose and cell death for “rapid” ablative and “slow” hyperthermic heating’. Int. J. Hyperth.36, 228–242 (2019). Google Scholar
- Hynynen, K., McDannold, N., Vykhodtseva, N. & Jolesz, F. A. Noninvasive MR imaging-guided focal opening of the blood–brain barrier in rabbits. Radiology220, 640–646 (2001). CASPubMedGoogle Scholar
- Sukovich, J. R. et al. In vivo histotripsy brain treatment. J. Neurosurg.131, 1331–1338 (2019). Google Scholar
- Lozano, A. M. et al. Deep brain stimulation: current challenges and future directions. Nat. Rev. Neurol.15, 148–160 (2019). PubMedPubMed CentralGoogle Scholar
- Deuschl, G. et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N. Engl. J. Med.355, 896–908 (2006). CASPubMedGoogle Scholar
- McDannold, N., Clement, G. T., Black, P., Jolesz, F. & Hynynen, K. Transcranial magnetic resonance imaging-guided focused ultrasound surgery of brain tumors: initial findings in 3 patients. Neurosurgery66, 323–332 (2010). PubMedPubMed CentralGoogle Scholar
- Coluccia, D. et al. First noninvasive thermal ablation of a brain tumor with MR-guided focused ultrasound. J. Ther. Ultrasound2, 17 (2014). PubMedPubMed CentralGoogle Scholar
- Jung, N. Y. et al. Factors related to successful energy transmission of focused ultrasound through a skull: a study in human cadavers and its comparison with clinical experiences. J. Korean Neurosurg. Soc.62, 712–722 (2019). PubMedPubMed CentralGoogle Scholar
- Benito-León, J. & Louis, E. D. Essential tremor: emerging views of a common disorder. Nat. Rev. Neurol.2, 666–678 (2006). Google Scholar
- Elble, R. J. The essential tremor syndromes. Curr. Opin. Neurol.29, 507–512 (2016). PubMedGoogle Scholar
- Elble, R. J. Mechanisms of deep brain stimulation for essential tremor. Brain137, 4–6 (2014). PubMedPubMed CentralGoogle Scholar
- Sharifi, S., Nederveen, A. J., Booij, J. & van Rootselaar, A.-F. Neuroimaging essentials in essential tremor: a systematic review. Neuroimage Clin.5, 217–231 (2014). PubMedPubMed CentralGoogle Scholar
- Dallapiazza, R. F. et al. Outcomes from stereotactic surgery for essential tremor. J. Neurol. Neurosurg. Psychiatry90, 474–482 (2019). PubMedGoogle Scholar
- Lipsman, N. et al. MR-guided focused ultrasound thalamotomy for essential tremor: a proof-of-concept study. Lancet Neurol.12, 462–468 (2013). PubMedGoogle Scholar
- Elias, W. J. et al. A pilot study of focused ultrasound thalamotomy for essential tremor. N. Engl. J. Med.369, 640–648 (2013). CASPubMedGoogle Scholar
- Scantlebury, N. et al. Change in some quality of life domains mimics change in tremor severity after ultrasound thalamotomy. Mov. Disord.34, 1400–1401 (2019). PubMedGoogle Scholar
- Chang, J. W. et al. A prospective trial of magnetic resonance guided focused ultrasound thalamotomy for essential tremor: results at the 2-year follow-up. Ann. Neurol.83, 107–114 (2017). Google Scholar
- Meng, Y. et al. Magnetic resonance-guided focused ultrasound thalamotomy for treatment of essential tremor: a 2-year outcome study: MRgFUS thalamotomy for ET: 2-year outcome. Mov. Disord.33, 1647–1650 (2018). PubMedGoogle Scholar
- Park, Y.-S., Jung, N. Y., Na, Y. C. & Chang, J. W. Four-year follow-up results of magnetic resonance-guided focused ultrasound thalamotomy for essential tremor. Mov. Disord.34, 727–734 (2019). PubMedGoogle Scholar
- Weidman, E. K., Kaplitt, M. G., Strybing, K. & Chazen, J. L. Repeat magnetic resonance imaging-guided focused ultrasound thalamotomy for recurrent essential tremor: case report and review of MRI findings. J. Neurosurg.132, 211–216 (2020). Google Scholar
- Fishman, P. S. et al. Neurological adverse event profile of magnetic resonance imaging-guided focused ultrasound thalamotomy for essential tremor. Mov. Disord.33, 843–847 (2018). PubMedGoogle Scholar
- Boutet, A. et al. Focused ultrasound thalamotomy location determines clinical benefits in patients with essential tremor. Brain141, 3405–3414 (2018). PubMedGoogle Scholar
- Pineda-Pardo, J. A. et al. Transcranial magnetic resonance-guided focused ultrasound thalamotomy in essential tremor: a comprehensive lesion characterization. Neurosurgery87, 256–265 (2019). Google Scholar
- Wintermark, M. et al. Thalamic connectivity in patients with essential tremor treated with MR imaging-guided focused ultrasound: in vivo fiber tracking by using diffusion-tensor MR imaging. Radiology272, 202–209 (2014). PubMedGoogle Scholar
- Pineda-Pardo, J. A. et al. Microstructural changes of the dentato-rubro-thalamic tract after transcranial MR guided focused ultrasound ablation of the posteroventral VIM in essential tremor. Hum. Brain Mapp.40, 2933–2942 (2019). PubMedPubMed CentralGoogle Scholar
- Pouratian, N., Baltuch, G., Elias, W. J. & Gross, R. American Society for Stereotactic and Functional Neurosurgery position statement on magnetic resonance-guided focused ultrasound for the management of essential tremor. Neurosurgery87, E126–E129 (2020). PubMedGoogle Scholar
- Ravikumar, V. K. et al. Cost-effectiveness of focused ultrasound, radiosurgery, and DBS for essential tremor. Mov. Disord.32, 1165–1173 (2017). PubMedGoogle Scholar
- Li, C. et al. Cost-effectiveness of magnetic resonance-guided focused ultrasound for essential tremor. Mov. Disord.34, 735–743 (2019). PubMedGoogle Scholar
- Horisawa, S. et al. A single case of MRI-guided focused ultrasound ventro-oral thalamotomy for musician’s dystonia. J. Neurosurg.131, 384–386 (2018). PubMedGoogle Scholar
- Meng, Y., Suppiah, S., Scantlebury, N., Lipsman, N. & Schwartz, M. L. Treatment of a patient with task-specific writing tremor using magnetic resonance-guided focused ultrasound. Can. J. Neurol. Sci.45, 474–477 (2018). PubMedGoogle Scholar
- Fasano, A. et al. MRI-guided focused ultrasound thalamotomy in non-ET tremor syndromes. Neurology89, 771–775 (2017). PubMedGoogle Scholar
- Poewe, W. et al. Parkinson disease. Nat. Rev. Dis. Primers3, 17013 (2017). PubMedGoogle Scholar
- Kalia, S. K., Sankar, T. & Lozano, A. M. Deep brain stimulation for Parkinson’s disease and other movement disorders. Curr. Opin. Neurol.26, 374–380 (2013). PubMedGoogle Scholar
- Prasad, S. et al. Spinal cord stimulation for very advanced Parkinson’s disease: a 1-year prospective trial. Mov. Disord.35, 1082–1083 (2020). PubMedGoogle Scholar
- Stefani, A. et al. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease. Brain130, 1596–1607 (2007). PubMedGoogle Scholar
- López-Azcárate, J. et al. Coupling between beta and high-frequency activity in the human subthalamic nucleus may be a pathophysiological mechanism in Parkinson’s disease. J. Neurosci.30, 6667–6677 (2010). PubMedPubMed CentralGoogle Scholar
- Martínez-Fernández, R. et al. Focused ultrasound subthalamotomy in patients with asymmetric Parkinson’s disease: a pilot study. Lancet Neurol.17, 54–63 (2018). This small open-label trial showed that unilateral MRgFUS subthalamotomy was technically feasible, effective and associated with a relatively low risk of hemichorea–ballism. PubMedGoogle Scholar
- Jung, N. Y. et al. The efficacy and limits of magnetic resonance-guided focused ultrasound pallidotomy for Parkinson’s disease: a phase I clinical trial. J. Neurosurg.130, 1853–1861 (2018). Google Scholar
- Gallay, M. N. et al. MRgFUS pallidothalamic tractotomy for chronic therapy-resistant Parkinson’s disease in 51 consecutive patients: single center experience. Front. Surg.6, 76 (2020). PubMedPubMed CentralGoogle Scholar
- Alvarez, L. Bilateral subthalamotomy in Parkinson’s disease: initial and long-term response. Brain128, 570–583 (2005). CASPubMedGoogle Scholar
- Meng, Y. et al. Cost-effectiveness analysis of MR-guided focused ultrasound thalamotomy for tremor-dominant Parkinson’s disease. J. Neurosurg.https://doi.org/10.3171/2020.5.JNS20692 (2020). ArticlePubMedGoogle Scholar
- Stein, D. J. et al. Obsessive–compulsive disorder. Nat. Rev. Dis. Primers5, 52 (2019). PubMedPubMed CentralGoogle Scholar
- Garnaat, S. L. et al. Who qualifies for deep brain stimulation for OCD? Data from a naturalistic clinical sample. J. Neuropsychiatry Clin. Neurosci.26, 81–86 (2014). PubMedPubMed CentralGoogle Scholar
- Pauls, D. L., Abramovitch, A., Rauch, S. L. & Geller, D. A. Obsessive–compulsive disorder: an integrative genetic and neurobiological perspective. Nat. Rev. Neurosci.15, 410–424 (2014). CASPubMedGoogle Scholar
- Whiteside, S. P., Port, J. D. & Abramowitz, J. S. A meta-analysis of functional neuroimaging in obsessive–compulsive disorder. Psychiatry Res.132, 69–79 (2004). PubMedGoogle Scholar
- Hamani, C. et al. Deep brain stimulation for obsessive–compulsive disorder. Neurosurgery75, 327–333 (2014). PubMedGoogle Scholar
- Mallet, L. et al. Subthalamic nucleus stimulation in severe obsessive–compulsive disorder. N. Engl. J. Med.359, 2121–2134 (2008). CASPubMedGoogle Scholar
- Denys, D. et al. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive–compulsive disorder. Arch. Gen. Psychiatry67, 1061–1068 (2010). PubMedGoogle Scholar
- Rück, C. et al. Capsulotomy for obsessive–compulsive disorder: long-term follow-up of 25 patients. Arch. Gen. Psychiatry65, 914–921 (2008). PubMedGoogle Scholar
- Alonso, P. et al. Deep brain stimulation for obsessive–compulsive disorder: a meta-analysis of treatment outcome and predictors of response. PLoS ONE10, e0133591 (2015). PubMedPubMed CentralGoogle Scholar
- Jung, H. H. et al. Bilateral thermal capsulotomy with MR-guided focused ultrasound for patients with treatment-refractory obsessive–compulsive disorder: a proof-of-concept study. Mol. Psychiatry20, 1205–1211 (2015). The first use of MRgFUS thermoablation for bilateral anterior capsulotomy to treat a psychiatric disorder. CASPubMedGoogle Scholar
- Kim, S. J. et al. A study of novel bilateral thermal capsulotomy with focused ultrasound for treatment-refractory obsessive–compulsive disorder: 2-year follow-up. J. Psychiatry Neurosci.43, 170188 (2018). PubMedGoogle Scholar
- Brown, L. T. et al. Dorsal anterior cingulotomy and anterior capsulotomy for severe, refractory obsessive–compulsive disorder: a systematic review of observational studies. J. Neurosurg.124, 77–89 (2016). PubMedGoogle Scholar
- Davidson, B. et al. Magnetic resonance-guided focused ultrasound capsulotomy for refractory obsessive compulsive disorder and major depressive disorder: clinical and imaging results from two phase I trials. Mol. Psychiatry25, 1946–1957 (2020). PubMedGoogle Scholar
- Otte, C. et al. Major depressive disorder. Nat. Rev. Dis. Primers2, 16065 (2016). PubMedGoogle Scholar
- Kim, M., Kim, C.-H., Jung, H. H., Kim, S. J. & Chang, J. W. Treatment of major depressive disorder via magnetic resonance-guided focused ultrasound surgery. Biol. Psychiatry83, e17–e18 (2018). PubMedGoogle Scholar
- Treede, R.-D. et al. A classification of chronic pain for ICD-11. Pain156, 1003–1007 (2015). PubMedPubMed CentralGoogle Scholar
- The Lancet Neurology. Novel ways to manage chronic pain are needed. Lancet Neurol.17, 829 (2018). CASPubMedGoogle Scholar
- Burchiel, K. J. & Raslan, A. M. Contemporary concepts of pain surgery. J. Neurosurg.130, 1039–1049 (2019). PubMedGoogle Scholar
- Martin, E., Jeanmonod, D., Morel, A., Zadicario, E. & Werner, B. High-intensity focused ultrasound for noninvasive functional neurosurgery. Ann. Neurol.66, 858–861 (2009). The first report of incisionless surgery using MRgFUS thermoablation in humans, undertaken in patients with chronic pain. PubMedGoogle Scholar
- Clary, A., Tyler, W. J. & Wetmore, D. Z. Abstract #45: ultrasound neuromodulation for the treatment of peripheral nerve compression syndromes. Brain Stimul.12, e16 (2019). Google Scholar
- Sweeney, M. D., Sagare, A. P. & Zlokovic, B. V. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol.14, 133–150 (2018). CASPubMedPubMed CentralGoogle Scholar
- Obermeier, B., Verma, A. & Ransohoff, R. M. The blood–brain barrier. Handb. Clin. Neurol.133, 39–59 (2016). PubMedGoogle Scholar
- Galea, I., Bechmann, I. & Perry, V. H. What is immune privilege (not)? Trends Immunol.28, 12–18 (2007). CASPubMedGoogle Scholar
- van Tellingen, O. et al. Overcoming the blood–brain tumor barrier for effective glioblastoma treatment. Drug Resist. Updat.19, 1–12 (2015). PubMedGoogle Scholar
- Arvanitis, C. D., Ferraro, G. B. & Jain, R. K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Rev. Cancer20, 26–41 (2020). CASPubMedGoogle Scholar
- Garbuzova-Davis, S., Thomson, A., Kurien, C., Shytle, R. D. & Sanberg, P. R. Potential new complication in drug therapy development for amyotrophic lateral sclerosis. Expert Rev. Neurother.16, 1397–1405 (2016). CASPubMedPubMed CentralGoogle Scholar
- Pardridge, W. M. The blood–brain barrier: bottleneck in brain drug development. NeuroRX2, 3–14 (2005). PubMedPubMed CentralGoogle Scholar
- Jablonski, M. R. et al. Inhibiting drug efflux transporters improves efficacy of ALS therapeutics. Ann. Clin. Transl. Neurol.1, 996–1005 (2014). CASPubMedPubMed CentralGoogle Scholar
- Meng, Y. et al. Safety and efficacy of focused ultrasound induced blood–brain barrier opening, an integrative review of animal and human studies. J. Control. Release309, 25–36 (2019). CASPubMedGoogle Scholar
- O’Reilly, M. A., Waspe, A. C., Chopra, R. & Hynynen, K. MRI-guided disruption of the blood–brain barrier using transcranial focused ultrasound in a rat model. J. Vis. Exp.61, 3555 (2012). Google Scholar
- Pelekanos, M. et al. Establishing sheep as an experimental species to validate ultrasound-mediated blood–brain barrier opening for potential therapeutic interventions. Theranostics8, 2583–2602 (2018). CASPubMedPubMed CentralGoogle Scholar
- Kovacs, Z. I. et al. Disrupting the blood–brain barrier by focused ultrasound induces sterile inflammation. Proc. Natl Acad. Sci. USA114, E75–E84 (2017). CASPubMedGoogle Scholar
- Poon, C. T. et al. Time course of focused ultrasound effects on β-amyloid plaque pathology in the TgCRND8 mouse model of Alzheimer’s disease. Sci. Rep.8, 14061 (2018). PubMedPubMed CentralGoogle Scholar
- Jordão, J. F. et al. Amyloid-β plaque reduction, endogenous antibody delivery and glial activation by brain-targeted, transcranial focused ultrasound. Exp. Neurol.248, 16–29 (2013). PubMedPubMed CentralGoogle Scholar
- McMahon, D., Bendayan, R. & Hynynen, K. Acute effects of focused ultrasound-induced increases in blood–brain barrier permeability on rat microvascular transcriptome. Sci. Rep.7, 45657 (2017). CASPubMedPubMed CentralGoogle Scholar
- McMahon, D. & Hynynen, K. Acute inflammatory response following increased blood–brain barrier permeability induced by focused ultrasound is dependent on microbubble dose. Theranostics7, 3989–4000 (2017). CASPubMedPubMed CentralGoogle Scholar
- Olumolade, O. O., Wang, S., Samiotaki, G. & Konofagou, E. E. Longitudinal motor and behavioral assessment of blood–brain barrier opening with transcranial focused ultrasound. Ultrasound Med. Biol.42, 2270–2282 (2016). PubMedPubMed CentralGoogle Scholar
- Horodyckid, C. et al. Safe long-term repeated disruption of the blood–brain barrier using an implantable ultrasound device: a multiparametric study in a primate model. J. Neurosurg.126, 1351–1361 (2017). PubMedGoogle Scholar
- Kinoshita, M., McDannold, N., Jolesz, F. A. & Hynynen, K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood–brain barrier disruption. Proc. Natl Acad. Sci. USA103, 11719–11723 (2006). CASPubMedGoogle Scholar
- Wu, S.-K. et al. Characterization of different microbubbles in assisting focused ultrasound-induced blood–brain barrier opening. Sci. Rep.7, 46689 (2017). CASPubMedPubMed CentralGoogle Scholar
- McDannold, N., Vykhodtseva, N. & Hynynen, K. Effects of acoustic parameters and ultrasound contrast agent dose on focused-ultrasound induced blood–brain barrier disruption. Ultrasound Med. Biol.34, 930–937 (2008). PubMedPubMed CentralGoogle Scholar
- Chen, H. & Konofagou, E. E. The size of blood–brain barrier opening induced by focused ultrasound is dictated by the acoustic pressure. J. Cereb. Blood Flow Metab.34, 1197–1204 (2014). CASPubMedPubMed CentralGoogle Scholar
- Jordão, J. F. et al. Antibodies targeted to the brain with image-guided focused ultrasound reduces amyloid-beta plaque load in the TgCRND8 mouse model of Alzheimer’s disease. PLoS ONE5, e10549 (2010). PubMedPubMed CentralGoogle Scholar
- Kobus, T., Zervantonakis, I. K., Zhang, Y. & McDannold, N. J. Growth inhibition in a brain metastasis model by antibody delivery using focused ultrasound-mediated blood–brain barrier disruption. J. Control. Release238, 281–288 (2016). CASPubMedPubMed CentralGoogle Scholar
- Liu, H.-L. et al. Focused ultrasound enhances central nervous system delivery of bevacizumab for malignant glioma treatment. Radiology281, 99–108 (2016). PubMedGoogle Scholar
- Alecou, T., Giannakou, M. & Damianou, C. Amyloid β plaque reduction with antibodies crossing the blood–brain barrier, which was opened in 3 sessions of focused ultrasound in a rabbit model. J. Ultrasound Med.36, 2257–2270 (2017). PubMedGoogle Scholar
- Alli, S. et al. Brainstem blood brain barrier disruption using focused ultrasound: a demonstration of feasibility and enhanced doxorubicin delivery. J. Control. Release281, 29–41 (2018). CASPubMedPubMed CentralGoogle Scholar
- Coluccia, D. et al. Enhancing glioblastoma treatment using cisplatin-gold-nanoparticle conjugates and targeted delivery with magnetic resonance-guided focused ultrasound. Nanomedicine14, 1137–1148 (2018). CASPubMedGoogle Scholar
- Thévenot, E. et al. Targeted delivery of self-complementary adeno-associated virus serotype 9 to the brain, using magnetic resonance imaging-guided focused ultrasound. Hum. Gene Ther.23, 1144–1155 (2012). PubMedPubMed CentralGoogle Scholar
- Burgess, A. et al. Targeted delivery of neural stem cells to the brain using MRI-guided focused ultrasound to disrupt the blood–brain barrier. PLoS ONE6, e27877 (2011). CASPubMedPubMed CentralGoogle Scholar
- Alkins, R. et al. Focused ultrasound delivers targeted immune cells to metastatic brain tumors. Cancer Res.73, 1892–1899 (2013). CASPubMedPubMed CentralGoogle Scholar
- Alkins, R., Burgess, A., Kerbel, R., Wels, W. S. & Hynynen, K. Early treatment of HER2-amplified brain tumors with targeted NK-92 cells and focused ultrasound improves survival. Neuro Oncol.18, 974–981 (2016). CASPubMedPubMed CentralGoogle Scholar
- Noroozian, Z. et al. MRI-guided focused ultrasound for targeted delivery of rAAV to the brain. Methods Mol. Biol.1950, 177–197 (2019). CASPubMedPubMed CentralGoogle Scholar
- Lipsman, N. et al. Blood–brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nat. Commun.9, 2336 (2018). The first report that transcranial FUS BBBO is safe in people with mild-to-moderate AD. PubMedPubMed CentralGoogle Scholar
- Pouliopoulos, A. N. et al. A clinical system for non-invasive blood–brain barrier opening using a neuronavigation-guided single-element focused ultrasound transducer. Ultrasound Med. Biol.46, 73–89 (2020). PubMedGoogle Scholar
- Asquier, N. et al. Blood–brain barrier disruption in humans using an implantable ultrasound device: quantification with MR images and correlation with local acoustic pressure. J. Neurosurg.132, 875–883 (2019). Google Scholar
- Beccaria, K. et al. Blood–brain barrier disruption with low-intensity pulsed ultrasound for the treatment of pediatric brain tumors: a review and perspectives. Neurosurg. Focus.48, E10 (2020). PubMedGoogle Scholar
- Alzheimer’s Association. 2017 Alzheimer’s disease facts and figures. Alzheimers Dement.13, 325–373 (2017). Google Scholar
- Masters, C. L. et al. Alzheimer’s disease. Nat. Rev. Dis. Primers1, 15056 (2015). PubMedGoogle Scholar
- Greenberg, S. M. et al. Cerebral amyloid angiopathy and Alzheimer disease — one peptide, two pathways. Nat. Rev. Neurol.16, 30–42 (2020). CASPubMedGoogle Scholar
- Nisbet, R. M. et al. Combined effects of scanning ultrasound and a tau-specific single chain antibody in a tau transgenic mouse model. Brain140, 1220–1230 (2017). PubMedPubMed CentralGoogle Scholar
- Xhima, K. et al. Focused ultrasound delivery of a selective TrkA agonist rescues cholinergic function in a mouse model of Alzheimer’s disease. Sci. Adv.6, eaax6646 (2020). CASPubMedPubMed CentralGoogle Scholar
- Burgess, A. et al. Alzheimer disease in a mouse model: MR imaging-guided focused ultrasound targeted to the hippocampus opens the blood–brain barrier and improves pathologic abnormalities and behavior. Radiology273, 736–745 (2014). PubMedPubMed CentralGoogle Scholar
- Leinenga, G. & Götz, J. Scanning ultrasound removes amyloid-β and restores memory in an Alzheimer’s disease mouse model. Sci. Transl. Med.7, 278ra33 (2015). PubMedGoogle Scholar
- Leinenga, G. & Götz, J. Safety and efficacy of scanning ultrasound treatment of aged APP23 mice. Front. Neurosci.12, 55 (2018). PubMedPubMed CentralGoogle Scholar
- Scarcelli, T. et al. Stimulation of hippocampal neurogenesis by transcranial focused ultrasound and microbubbles in adult mice. Brain Stimul.7, 304–307 (2014). PubMedPubMed CentralGoogle Scholar
- Mooney, S. J. et al. Focused ultrasound-induced neurogenesis requires an increase in blood–brain barrier permeability. PLoS ONE11, e0159892 (2016). PubMedPubMed CentralGoogle Scholar
- Nation, D. A. et al. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med.25, 270–276 (2019). CASPubMedPubMed CentralGoogle Scholar
- Rezai, A. R. et al. Noninvasive hippocampal blood−brain barrier opening in Alzheimer’s disease with focused ultrasound. Proc. Natl Acad. Sci. USA117, 9180–9182 (2020). CASPubMedGoogle Scholar
- Panza, F., Lozupone, M., Logroscino, G. & Imbimbo, B. P. A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nat. Rev. Neurol.15, 73–88 (2019). PubMedGoogle Scholar
- Hardiman, O. et al. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers3, 17085 (2017). PubMedGoogle Scholar
- Hobson, E. V. & McDermott, C. J. Supportive and symptomatic management of amyotrophic lateral sclerosis. Nat. Rev. Neurol.12, 526–538 (2016). CASPubMedGoogle Scholar
- Hetz, C. & Saxena, S. ER stress and the unfolded protein response in neurodegeneration. Nat. Rev. Neurol.13, 477–491 (2017). CASPubMedGoogle Scholar
- Lacomblez, L., Bensimon, G., Meininger, V., Leigh, P. N. & Guillet, P. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Lancet347, 1425–1431 (1996). CASPubMedGoogle Scholar
- Edaravone (MCI-186) ALS 19 Study Group. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol.16, 505–512 (2017). Google Scholar
- Geevasinga, N., Menon, P., Özdinler, P. H., Kiernan, M. C. & Vucic, S. Pathophysiological and diagnostic implications of cortical dysfunction in ALS. Nat. Rev. Neurol.12, 651–661 (2016). CASPubMedGoogle Scholar
- Thomsen, G. M. et al. Delayed disease onset and extended survival in the SOD1G93A rat model of amyotrophic lateral sclerosis after suppression of mutant SOD1 in the motor cortex. J. Neurosci.34, 15587–15600 (2014). PubMedPubMed CentralGoogle Scholar
- Thomsen, G. M. et al. Transplantation of neural progenitor cells expressing glial cell line-derived neurotrophic factor into the motor cortex as a strategy to treat amyotrophic lateral sclerosis. Stem Cell36, 1122–1131 (2018). CASGoogle Scholar
- Abrahao, A. et al. First-in-human trial of blood–brain barrier opening in amyotrophic lateral sclerosis using MR-guided focused ultrasound. Nat. Commun.10, 4373 (2019). The first study of transcranial FUS BBBO in eloquent cortex in people with ALS, showing that the procedure was safe and technically successful. PubMedPubMed CentralGoogle Scholar
- Axelsen, T. M. & Woldbye, D. P. D. Gene therapy for Parkinson’s disease, an update. J. Parkinsons Dis.8, 195–215 (2018). PubMedPubMed CentralGoogle Scholar
- Xhima, K., Nabbouh, F., Hynynen, K., Aubert, I. & Tandon, A. Noninvasive delivery of an α-synuclein gene silencing vector with magnetic resonance-guided focused ultrasound. Mov. Disord.33, 1567–1579 (2018). CASPubMedPubMed CentralGoogle Scholar
- Fan, C.-H. et al. Noninvasive, targeted, and non-viral ultrasound-mediated GDNF-plasmid delivery for treatment of Parkinson’s disease. Sci. Rep.6, 19579 (2016). CASPubMedPubMed CentralGoogle Scholar
- Fan, C.-H., Lin, C.-Y., Liu, H.-L. & Yeh, C.-K. Ultrasound targeted CNS gene delivery for Parkinson’s disease treatment. J. Control. Release261, 246–262 (2017). CASPubMedGoogle Scholar
- Stupp, R. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med.352, 987–996 (2005). CASPubMedGoogle Scholar
- Mainprize, T. et al. Blood–brain barrier opening in primary brain tumors with non-invasive MR-guided focused ultrasound: a clinical safety and feasibility study. Sci. Rep.9, 321 (2019). PubMedPubMed CentralGoogle Scholar
- Idbaih, A. et al. Safety and feasibility of repeated and transient blood–brain barrier disruption by pulsed ultrasound in patients with recurrent glioblastoma. Clin. Cancer Res.25, 3793–3801 (2019). This study indicated that the use of an implanted ultrasound device to deliver repeated BBBO during carboplatin administration for recurrent glioblastoma is safe and potentially enhances progression-free survival. CASPubMedGoogle Scholar
- Razavi, S.-M. et al. Immune evasion strategies of glioblastoma. Front. Surg.3, 11 (2016). PubMedPubMed CentralGoogle Scholar
- Chen, P.-Y. et al. Focused ultrasound-induced blood–brain barrier opening to enhance interleukin-12 delivery for brain tumor immunotherapy: a preclinical feasibility study. J. Transl. Med.13, 93 (2015). PubMedPubMed CentralGoogle Scholar
- Sheybani, N. D., Witter, A. R., Stevens, A. D., Bullock, T. N. & Price, R. J. Focused ultrasound ablation as an immunomodulatory strategy for metastatic breast cancer therapy. J. Immunol.200, 178.39 (2018). Google Scholar
- Sperling, R. A. et al. Amyloid related imaging abnormalities (ARIA) in amyloid modifying therapeutic trials: recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dement.7, 367–385 (2011). PubMedPubMed CentralGoogle Scholar
- Schneider, S., Potthast, S., Komminoth, P., Schwegler, G. & Böhm, S. PD-1 checkpoint inhibitor associated autoimmune encephalitis. Case Rep. Oncol.10, 473–478 (2017). PubMedPubMed CentralGoogle Scholar
- Meng, Y. et al. Glymphatics visualization after focused ultrasound-induced blood–brain barrier opening in humans. Ann. Neurol.86, 975–980 (2019). Localized and transient BBB opening using FUS allowed the first in vivo visualization of the glymphatic system in humans. CASPubMedGoogle Scholar
- Engelhardt, B., Vajkoczy, P. & Weller, R. O. The movers and shapers in immune privilege of the CNS. Nat. Immunol.18, 123–131 (2017). CASPubMedGoogle Scholar
- Park, E.-J., Zhang, Y.-Z., Vykhodtseva, N. & McDannold, N. Ultrasound-mediated blood–brain/blood-tumor barrier disruption improves outcomes with trastuzumab in a breast cancer brain metastasis model. J. Control. Release163, 277–284 (2012). CASPubMedPubMed CentralGoogle Scholar
- Park, S. H. et al. Safety and feasibility of multiple blood–brain barrier disruptions for the treatment of glioblastoma in patients undergoing standard adjuvant chemotherapy. J. Neurosurg. https://doi.org/10.3171/2019.10.JNS192206 (2020).
- O’Reilly, M. A. et al. Preliminary investigation of focused ultrasound-facilitated drug delivery for the treatment of leptomeningeal metastases. Sci. Rep.8, 9013 (2018). PubMedPubMed CentralGoogle Scholar
- Blumberger, D. M. et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet391, 1683–1692 (2018). PubMedGoogle Scholar
- Deisseroth, K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat. Neurosci.18, 1213–1225 (2015). CASPubMedPubMed CentralGoogle Scholar
- Dallapiazza, R. F. et al. Noninvasive neuromodulation and thalamic mapping with low-intensity focused ultrasound. J. Neurosurg.128, 875–884 (2018). PubMedGoogle Scholar
- Folloni, D. et al. Manipulation of subcortical and deep cortical activity in the primate brain using transcranial focused ultrasound stimulation. Neuron101, 1109–1116.e5 (2019). CASPubMedPubMed CentralGoogle Scholar
- Nicodemus, N. E. et al. Focused transcranial ultrasound for treatment of neurodegenerative dementia. Alzheimers Dement.5, 374–381 (2019). Google Scholar
- Legon, W. et al. Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nat. Neurosci.17, 322–329 (2014). CASPubMedGoogle Scholar
- Verhagen, L. et al. Offline impact of transcranial focused ultrasound on cortical activation in primates. eLife8, e40541 (2019). Demonstration that the neuromodulatory effect of FUS extends beyond the immediate period of sonication, which increases the translational potential of this technology. PubMedPubMed CentralGoogle Scholar
- Khalighinejad, N. et al. A basal forebrain–cingulate circuit in macaques decides it is time to act. Neuron105, 370–384.e8 (2020). CASPubMedPubMed CentralGoogle Scholar
- Wattiez, N. et al. Transcranial ultrasonic stimulation modulates single-neuron discharge in macaques performing an antisaccade task. Brain Stimul.10, 1024–1031 (2017). PubMedGoogle Scholar
- Younan, Y. et al. Influence of the pressure field distribution in transcranial ultrasonic neurostimulation. Med. Phys.40, 082902 (2013). PubMedGoogle Scholar
- Sato, T., Shapiro, M. G. & Tsao, D. Y. Ultrasonic neuromodulation causes widespread cortical activation via an indirect auditory mechanism. Neuron98, 1031–1041.e5 (2018). CASPubMedGoogle Scholar
- Guo, H. et al. Ultrasound produces extensive brain activation via a cochlear pathway. Neuron98, 1020–1030.e4 (2018). CASPubMedGoogle Scholar
- Constans, C., Mateo, P., Tanter, M. & Aubry, J.-F. Potential impact of thermal effects during ultrasonic neurostimulation: retrospective numerical estimation of temperature elevation in seven rodent setups. Phys. Med. Biol.63, 025003 (2018). PubMedGoogle Scholar
- Deffieux, T. et al. Low-intensity focused ultrasound modulates monkey visuomotor behavior. Curr. Biol.23, 2430–2433 (2013). CASPubMedGoogle Scholar
- Yoon, K. et al. Effects of sonication parameters on transcranial focused ultrasound brain stimulation in an ovine model. PLoS ONE14, e0224311 (2019). CASPubMedPubMed CentralGoogle Scholar
- Darrow, D. P., O’Brien, P., Richner, T. J., Netoff, T. I. & Ebbini, E. S. Reversible neuroinhibition by focused ultrasound is mediated by a thermal mechanism. Brain Stimul.12, 1439–1447 (2019). PubMedPubMed CentralGoogle Scholar
- Oh, S.-J. et al. Ultrasonic neuromodulation via astrocytic TRPA1. Curr. Biol.29, 3386–3401.e8 (2019). CASPubMedGoogle Scholar
- Chen, S.-G. et al. Transcranial focused ultrasound pulsation suppresses pentylenetetrazol induced epilepsy in vivo. Brain Stimul.13, 35–46 (2020). PubMedGoogle Scholar
- Cui, Z. et al. Enhanced neuronal activity in mouse motor cortex with microbubbles’ oscillations by transcranial focused ultrasound stimulation. Ultrason. Sonochem.59, 104745 (2019). CASPubMedGoogle Scholar
- Cho, H. et al. Localized down-regulation of P-glycoprotein by focused ultrasound and microbubbles induced blood–brain barrier disruption in rat brain. Sci. Rep.6, 31201 (2016). CASPubMedPubMed CentralGoogle Scholar
- Meng, Y. et al. Resting state functional connectivity changes after MR-guided focused ultrasound mediated blood–brain barrier opening in patients with Alzheimer’s disease. Neuroimage200, 275–280 (2019). PubMedGoogle Scholar
- Todd, N., Zhang, Y., Livingstone, M., Borsook, D. & McDannold, N. The neurovascular response is attenuated by focused ultrasound-mediated disruption of the blood–brain barrier. Neuroimage201, 116010 (2019). PubMedPubMed CentralGoogle Scholar
- Wang, Z., Yan, J., Wang, X., Yuan, Y. & Li, X. Transcranial ultrasound stimulation directly influences the cortical excitability of the motor cortex in Parkinsonian mice. Mov. Disord.35, 693–698 (2020). PubMedGoogle Scholar
- Lee, W. et al. Transcranial focused ultrasound stimulation of human primary visual cortex. Sci. Rep.6, 34026 (2016). CASPubMedPubMed CentralGoogle Scholar
- Salminen-Vaparanta, N., Noreika, V., Revonsuo, A., Koivisto, M. & Vanni, S. Is selective primary visual cortex stimulation achievable with TMS? Hum. Brain Mapp.33, 652–665 (2012). PubMedGoogle Scholar
- Beisteiner, R. et al. Transcranial pulse stimulation with ultrasound in Alzheimer’s disease — a new navigated focal brain therapy. Adv. Sci.7, 1902583 (2020). Google Scholar
- Cotero, V. et al. Noninvasive sub-organ ultrasound stimulation for targeted neuromodulation. Nat. Commun.10, 952 (2019). CASPubMedPubMed CentralGoogle Scholar
- Lea-Banks, H., O’Reilly, M. A., Hamani, C. & Hynynen, K. Localized anesthesia of a specific brain region using ultrasound-responsive barbiturate nanodroplets. Theranostics10, 2849–2858 (2020). CASPubMedPubMed CentralGoogle Scholar
- Todd, N. et al. Modulation of brain function by targeted delivery of GABA through the disrupted blood–brain barrier. Neuroimage189, 267–275 (2019). CASPubMedPubMed CentralGoogle Scholar
- Wu, X. et al. Sono-optogenetics facilitated by a circulation-delivered rechargeable light source for minimally invasive optogenetics. Proc. Natl Acad. Sci. USA116, 26332–26342 (2019). CASGoogle Scholar
- Wang, S. et al. Non-invasive, focused ultrasound-facilitated gene delivery for optogenetics. Sci. Rep.7, 39955 (2017). CASPubMedPubMed CentralGoogle Scholar
- Wang, J. B., Aryal, M., Zhong, Q., Vyas, D. B. & Airan, R. D. Noninvasive ultrasonic drug uncaging maps whole-brain functional networks. Neuron100, 728–738.e7 (2018). CASPubMedPubMed CentralGoogle Scholar
- Szablowski, J. O., Lee-Gosselin, A., Lue, B., Malounda, D. & Shapiro, M. G. Acoustically targeted chemogenetics for the non-invasive control of neural circuits. Nat. Biomed. Eng.2, 475–484 (2018). CASPubMedGoogle Scholar
- Constans, C. et al. Non-invasive ultrasonic modulation of visual evoked response by GABA delivery through the blood brain barrier. J. Control. Release318, 223–231 (2020). CASPubMedGoogle Scholar
- Frey, B. et al. Old and new facts about hyperthermia-induced modulations of the immune system. Int. J. Hyperth.28, 528–542 (2012). CASGoogle Scholar
- Cohen-Inbar, O., Xu, Z. & Sheehan, J. P. Focused ultrasound-aided immunomodulation in glioblastoma multiforme: a therapeutic concept. J. Ther. Ultrasound4, 2 (2016). PubMedPubMed CentralGoogle Scholar
- Man, J. et al. Hyperthermia sensitizes glioma stem-like cells to radiation by inhibiting AKT signaling. Cancer Res.75, 1760–1769 (2015). CASPubMedPubMed CentralGoogle Scholar
- Zhu, L. et al. Ultrasound hyperthermia technology for radiosensitization. Ultrasound Med. Biol.45, 1025–1043 (2019). PubMedPubMed CentralGoogle Scholar
- Yoshida, M. et al. Sonodynamic therapy for malignant glioma using 220-kHz transcranial magnetic resonance imaging-guided focused ultrasound and 5-aminolevulinic acid. Ultrasound Med. Biol.45, 526–538 (2019). PubMedGoogle Scholar
- Ricci, S. et al. Sonothrombolysis for acute ischaemic stroke. Cochrane Database Syst. Rev.10, CD008348 (2012). PubMedGoogle Scholar
- Alexandrov, A. V. et al. Safety and efficacy of sonothrombolysis for acute ischaemic stroke: a multicentre, double-blind, phase 3, randomised controlled trial. Lancet Neurol.18, 338–347 (2019). The addition of an operator-independent ultrasound device to alteplase was safe but did not improve functional outcomes at 90 days after ischaemic stroke. PubMedGoogle Scholar
- Alexandrov, A. V. et al. Endovascular equipoise shift in a phase III randomized clinical trial of sonothrombolysis for acute ischemic stroke. Ther. Adv. Neurol. Disord.12, 1756286419860652 (2019). PubMedPubMed CentralGoogle Scholar
- Gerhardson, T. et al. Histotripsy clot liquefaction in a porcine intracerebral hemorrhage model. Neurosurgery86, 429–436 (2020). PubMedGoogle Scholar
- Burgess, A. et al. High-intensity focused ultrasound (HIFU) for dissolution of clots in a rabbit model of embolic stroke. PLoS ONE7, e42311 (2012). CASPubMedPubMed CentralGoogle Scholar
- Chang, W. S. et al. Factors associated with successful magnetic resonance-guided focused ultrasound treatment: efficiency of acoustic energy delivery through the skull. J. Neurosurg.124, 411–416 (2016). PubMedGoogle Scholar
- D’Souza, M. et al. Impact of skull density ratio on efficacy and safety of magnetic resonance-guided focused ultrasound treatment of essential tremor. J. Neurosurg.132, 1392–1397 (2019). Google Scholar
- Schwartz, M. L. et al. Skull bone marrow injury caused by MR-guided focused ultrasound for cerebral functional procedures. J. Neurosurg.130, 758–762 (2019). Google Scholar
- Hughes, A. & Hynynen, K. Design of patient-specific focused ultrasound arrays for non-invasive brain therapy with increased trans-skull transmission and steering range. Phys. Med. Biol.62, L9–L19 (2017). PubMedPubMed CentralGoogle Scholar
- Arvanitis, C. D., Vykhodtseva, N., Jolesz, F., Livingstone, M. & McDannold, N. Cavitation-enhanced nonthermal ablation in deep brain targets: feasibility in a large animal model. J. Neurosurg.124, 1450–1459 (2015). PubMedPubMed CentralGoogle Scholar
- Macklin, R. The ethical problems with sham surgery in clinical research. N. Engl. J. Med.341, 992–996 (1999). CASPubMedGoogle Scholar
- Whone, A. et al. Randomized trial of intermittent intraputamenal glial cell line-derived neurotrophic factor in Parkinson’s disease. Brain142, 512–525 (2019). PubMedPubMed CentralGoogle Scholar
- Alonso, A. et al. Focal delivery of AAV2/1-transgenes into the rat brain by localized ultrasound-induced BBB opening. Mol. Ther. Nucleic Acids2, e73 (2013). PubMedPubMed CentralGoogle Scholar
Acknowledgements
We acknowledge Hang Yu Lin for her artistic contribution to the figures in this article. N. L. acknowledges and is grateful for the generous philanthropic gifts to the Sunnybrook Foundation, Sunnybrook Research Institute and the Harquail Centre for Neuromodulation as well as the support of the Focused Ultrasound Foundation.









